Bioavailability Analysis Services
Bioavailability measures the extent and efficiency of a drug's absorption and utilization in the body, encompassing processes such as absorption, distribution, metabolism, and excretion. The assessment of bioavailability is crucial for the rational use of drugs and the prediction of therapeutic effects.
As a model organism, C. elegans has the advantages of a short growth cycle, easy reproduction and cultivation, a well-characterized genetic background, and highly conservative evolution. These traits make it ideal for studying metabolic transformation and bioavailability of trace elements in the body. By using chromatographic methods to measure substances before and after nematode absorption, researchers can gain valuable insights into drug development, food safety, and environmental health.
Our Services
Our C. elegans bioavailability analysis service includes but is not limited to:
ØMetabolite Product Analysis
Evaluating drug biotransformation process by analyzing metabolites converted by enzymes and their contents after drugs or compounds are ingested by C. elegans.
ØBioequivalence Analysis
Analyzing the bioavailability of drug preparations is the same or comparable with different doses and routes of administration. Studying correlations between bioavailability and drug toxicity effects.
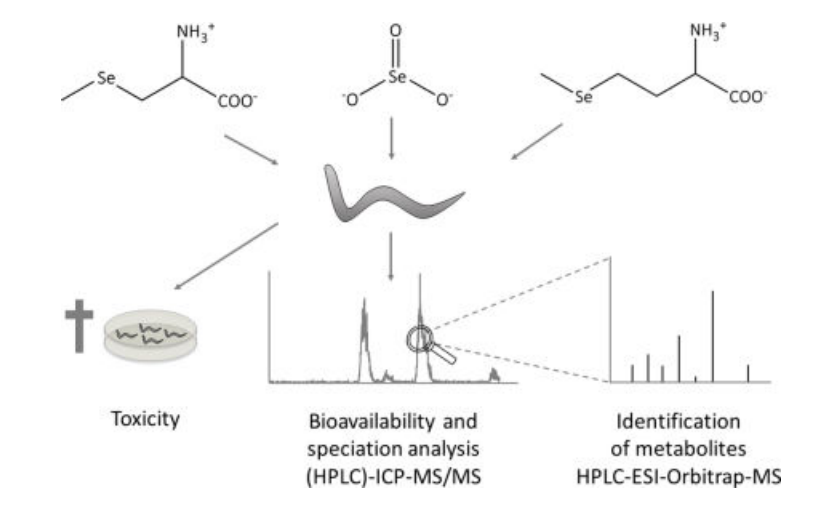
Figure 1. Determination of the bioavailability of different selenium compounds in Caenorhabditis elegans
(Rohn I, et al. Metallomics. 2018)
References
1. Rohn I, Marschall TA, Kroepfl N, Jensen KB, Aschner M, Tuck S, Kuehnelt D, Schwerdtle T, Bornhorst J. Selenium species-dependent toxicity, bioavailability and metabolic transformations in Caenorhabditis elegans . Metallomics . 2018 Jun 20 ;10 (6):818-827. doi : 10.1039/c8mt00066b.


