CRISPR/Cas9 Bacterial Gene Editing Service
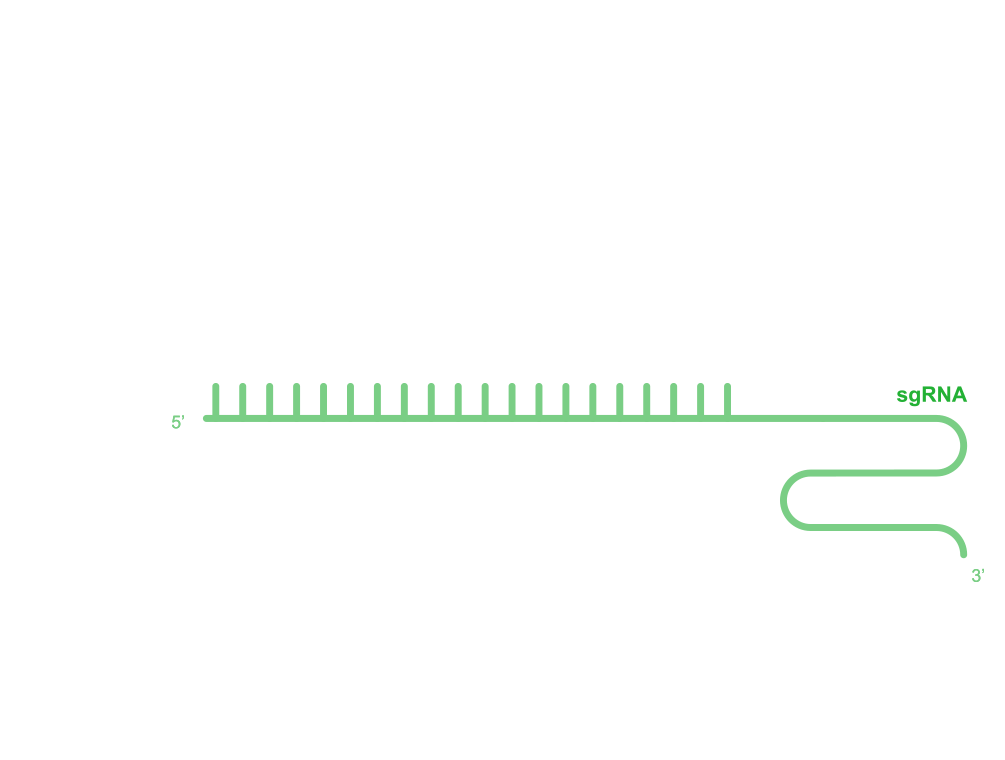
Thanks to the convenient CRISPR/Cas9 gene editing system, gene editing in species such as yeast, nematodes, drosophila, zebrafish, mice, and human cells has become more efficient. Currently, we have established a complete CRISPR/Cas9-mediated bacterial gene editing system (Figure 1) that can quickly and efficiently complete the following gene editing: precise sequence knockout, precise single nucleotide mutation, precise sequence insertion, precise short protein tag insertion, and precise sequence replacement.
Figure 1 CRISPR/Cas9 Gene Editing System
As a classic model organism for life science research, bacteria have wide applications in both basic and applied research.
In basic research:
- 1.Genomics research: E. coli is an ideal model for studying fundamental life processes, such as gene expression, DNA replication, transcription, and translation.
- 2.Genetics research: Bacteria have a simple genetic background, making gene mutation, screening, and localization easily.
- 3.Medical research: Mechanism research of how gut microbiota and their metabolites affect metabolism, health, and disease.
In applied research:
- 1.Recombinant protein expression: Using genome modified bacteria to produce various recombinant proteins, such as insulin, interferon, vaccine antigens, etc.
- 2.Metabolic engineering and synthetic biology: Modifying bacterial metabolic pathways to produce and synthesize industrial chemicals and biofuels, such as lactic acid, pigment ethanol, butanol, fatty acids, etc.
- 3. Environmental remediation and biopesticides: Creating genome edited beneficial bacteria for agriculture and fisheries, which can degrade pesticide residues, treat pests and diseases, or pathogens, such as Nocardia, Bacillus thuringiensis, etc.
- 4. Pharmaceutical development: Studying the pathogenic mechanisms of pathogenic bacteria or genetically modified pathogenic bacteria to help develop new antibiotics and treatments, such as Salmonella, Mycobacterium tuberculosis, etc.
- 5. Drug screening and toxicology testing: Using genetically modified pathogenic bacteria to rapidly and cost-effectively screen drugs and conduct toxicology tests.
Table 1 Service Process for Bacterial Transgenic Technology
| 1. Analysis of the needs | Free sample evaluation | 1-3 days |
| Strategy design | ||
| 2. Experimental procedure (varies depending on complexity) | Vector construction | 7-20 days |
| Transformation and screening | ||
| Sequence verification | ||
| 3. Deliverables | Mutated strain and experimental report | 5-7 days |
Table 2. Prices for Bacterial Gene Editing Services
| PID | Bacterial Gene Editing | Turnaround (weeks) | Price(US$)/strain | New Lab 30% OFF |
|---|---|---|---|---|
| A302 | Precise gene deletion | 4-5 | From 600 USD | From 420 USD |
| A303 | Precise nucleotide(s) changes | 4-5 | From 600 USD | From 420 USD |
| A304 | Precise sequence knock-in | 4-5 | From 799 USD | From 559 USD |
Deliverables:
- 1.Edited strains verified by sequencing
- 2.Sequencing report
How to Order:
Download the order form OR Start your inquiry
Have Other Questions?
You can always reach us at service@sunybiotech.com. Our customer service and editing experts will get back to you ASAP.