Age-dependent nuclear lipid droplet deposition can be used as a marker of aging
2023-04-02 15:48
Fat, it seems to be everywhere. The oil we use to cook our food, the marbling we see on a salmon steak, and perhaps the stubborn belly fat that won’t go away no matter how hard we tried. Biologically speaking, fat is nutrient and needed for the normal function of body. Lipid droplets are the main form of fat storage in cells, which provide energy and material basis for membranes synthesis and maintenance. Lipid droplets are also present in the nucleus, but their function remains unclear.
In the article “Age-dependent nuclear lipid droplet deposition is a cellular hallmark of aging in Caenorhabditis elegans”, the researchers went through and shared their findings on evaluating the accumulation of nuclear lipid droplets (nLDs) in C.elegans as they age. Different types of transgenic worms were recruited to establish the relationships between nLDs accrual and aging, and to explore its underlying mechanisms.
First and foremost, the researchers talked about the technology they applied to visualize and quantify nLDs. Third harmonic generation (THG) imagining technology was described as a non-invasive and label-free method for precise fat content identification in vivo at the microscopic level. With this method, combined with other tools such as two-photon-excitation fluorescence (TPEF), they were able to keep track of the fat content within the nuclear compartment of the worms expressing transgenic fluorescent reporters (LMN-1::GFP and EMR-1::mCherry). An age-dependent change can be detected by following the development of intestinal cells, with gradual increase of nLDs in both number and size as the worm aged from day 1 to 10 (Figure 1).
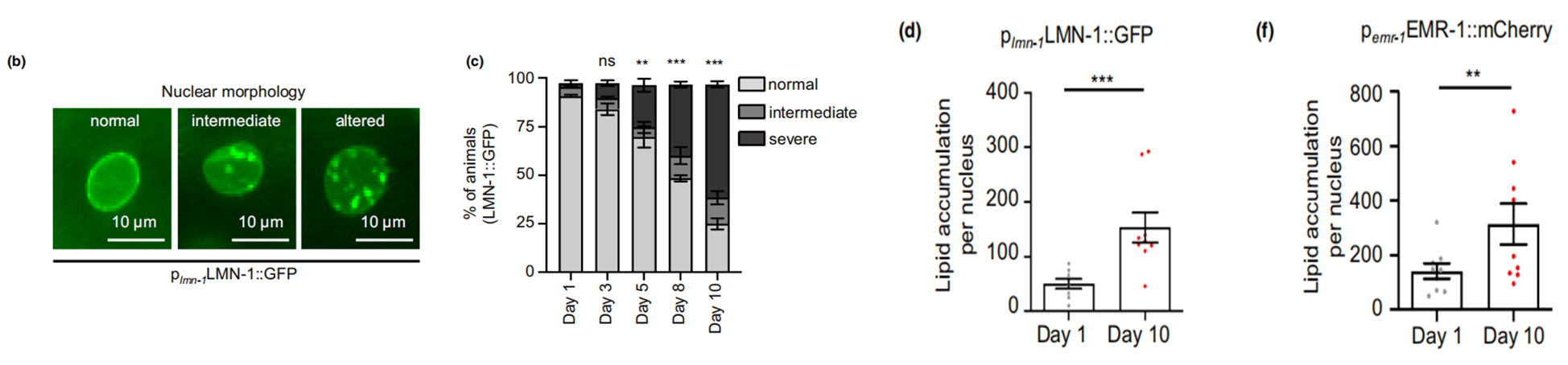
Figure 1. Age-dependent increase of fat content in the nucleus.
Previous researches with long-living mutant strains eat-2(ad465) and daf-2(e1370) has established that dietary restriction and inhibition of insulin signaling can alleviate age-dependent fat deposition. Based on this, the authors found that, as the worms aged, these mutant strains of nematodes also showed a lower amount of nuclear lipid content and a concomitant decrease in the size of the droplets, compared to the natural wild type worms (Figure 2). From there, they suggested that the lipid metabolism in the nuclear compartment of these mutant worms must have been enhanced, hence the reduced quantity and size of the nLDs. So, what about the mechanisms?
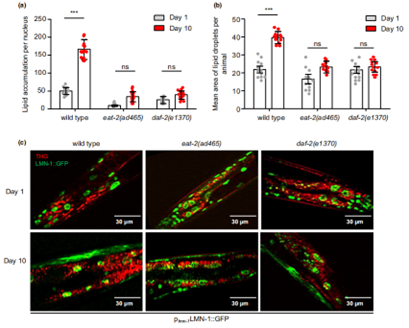
Figure 2. Lipid accumulation analysis in long-living mutant strains.
Fluorescent tags were used to track two proteins of interest, mCherry for the EMR-1 protein which is an inner nuclear membrane protein, and GFP for LMN-1 protein, the nuclear lamina protein. From the experiments, the researchers found a differential effect by aging on the level of these two proteins, which happened to be in correlation with the accumulation of nLDs. The protein level and accumulation of the EMR-1 protein remained rather stable in both of the mutant strains, while the LMN-1 levels are stable in eat-2(ad465) and display a slower accumulation rate in DAF-2 deficient animals during aging compared to the wild type. Therefore, it’s suggested that the pathway though which caloric restriction and low insulin signaling impact aging is probably to do with the regulation of the expression of LMN-1 proteins (Figure 3).
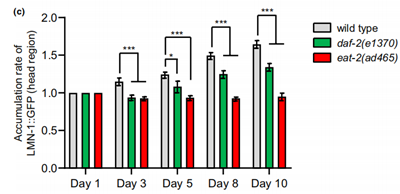
Figure 3. The accumulation of LMN-1 protein as the worms aged.
Overall, the study found that lipid droplets accumulate increasingly in the nuclear membrane during aging, but that interventions to promote longevity, such as low insulin signaling regulation and caloric restriction, can eliminate the rate of nuclear lipid accumulation and reduce the size of lipid droplets. C. elegans is a versatile genetic model that can be used as a screening platform to unravel novel tissue-specific modulators of nLDs distribution. Investigating further the molecular pathways that regulate nLDs formation and accrual will enlighten new avenues for therapeutic intervention strategies to tackle metabolic and age-associated diseases.
Reference:
Palikaras, K., Mari, M., Ploumi, C., Princz, A., Filippidis, G., & Tavernarakis, N. (2023). Age dependent nuclear lipid droplet deposition is a cellular hallmark of aging in Caenorhabditis elegans. Aging Cell, 00, e13788.
https://doi.org/10.1111/acel.13788





