Maillard reaction: What’s lurking beneath that tempting golden goodness?
2023-11-27 09:47
Anyone who is well-versed in the art of the kitchen would tell you something like this, “No browning, no flavor.” This refers to the Maillard reaction, a renowned chemical reaction discovered by French chemist L.C. Maillard. When glucose and glycine are heated, they undergo a reaction that results in the formation of brown pigments and also produces flavors. This reaction makes meat and vegetables more appetizing to us (verse from the "Kitchen Bible"). In fact, many home cooks and restaurant chefs are now opting for cooking methods that fully harness the power of the Maillard reaction, such as searing, roasting, broiling, and more.
However, wouldn't it be great if the Maillard reaction could also provide health benefits while enhancing the flavors of our meat? Unfortunately, current research seems to disagree. One such study, published in the journal eLife, looked at why we may need to be cautious about the Maillard reaction's effects (Methylglyoxal-derived hydroimidazolone, MG-H1, increases food intake by altering tyramine signaling via the GATA transcription factor ELT-3 in Caenorhabditis elegans). Scientists have found advanced glycation end-products (AGEs) to be among the end products of the Maillard reaction, along with many other toxic byproducts, including acrylamide. By employing the model organism C. elegans, the authors set out to explore how AGEs, MG-H1 typically, interfere with worm lifespan and healthspan, the potential pathways behind, and how it connects chronic diseases with obesity.
By employing a mutant strain of C. elegans lacking the glod-4 glyoxalase enzyme, which helps detoxify methylglyoxal (MGO) into non-toxic lactate, the researchers were able to demonstrate that AGEs could increase food intake and food-seeking behaviors in C. elegans. The glod-4 mutant worms displayed higher endogenous AGEs accumulations due to their dysfunctional glyoxalase production. Consequently, they were found to have an increased food clearance rate and higher pharyngeal pumping rate, which are two biomarkers used to assess nutrient intake, compared to the wild type (Figure 1). Simply put, they were eating more.
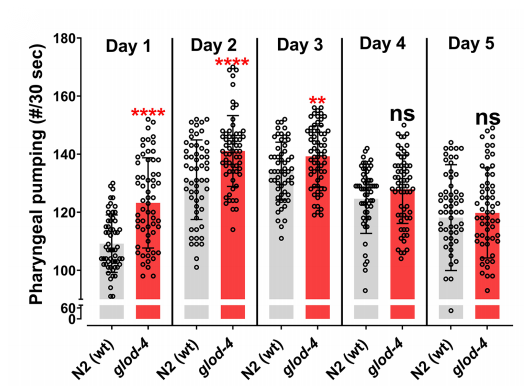
Figure 1. Mutant worms with dysfunctional glyoxalase production appeared to have a higher food intake compared to the wild type control.
As AGEs encompass a wide range of molecules, the researchers further investigated which specific compounds were responsible for the observed phenomenon. They explored a series of possible molecules, such as MG-H1, CEL, CML, and F-ly, and were able to identify MG-H1 and CEL as potential MGO-derived AGEs that increase feeding in C. elegans. By treating the wild type worms with 150uM MG-H1, the researchers observed an increase in their pharyngeal pumping rate within a 24-hour time frame (Figure 2). Hence, it seemed that the same effect was present when AGEs were fed exogenously, a scenario mimicking our daily consumption of browned food.
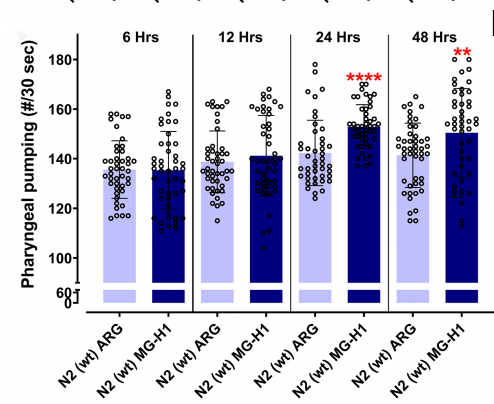
Figure 2. Wild type animals treated with MG-H1 after 24h were found to have an increased PPR.
Interestingly, the researchers also found that adding MG-H1 to the OP50 lawn (worm food) made it more appealing to the wild type worms. This resulted in a higher percentage of worms reaching for the food compared to the trials with other additives. Well, it seems that this magic trick not only fools us humans but also deceives tiny nematode worms.
Next up, it’s time to examine the potential signaling pathways behind it. Through a series of tests and screenings, the authors identified the role of a compound called tyramine in regulating MG-H1-mediated feeding behavior. Tyramine is a neurotransmitter, and the tdc-1 mutant worms (those that were genetically edited to have a dysfunctional tyramine circuit) appeared to exhibit a suppressed feeding rate when AGEs accumulated (Figure 3). Downstream, the authors investigated the receptors for tyramine and found that mutations in the ser-2 and tyra-2 genes (genes coding for tyramine and octopamine receptors) also had the effect of suppressing food intake. Therefore, it is sufficiently demonstrated that tyramine plays a crucial role, both upstream and downstream, in regulating MG-H1-mediated feeding behavior.
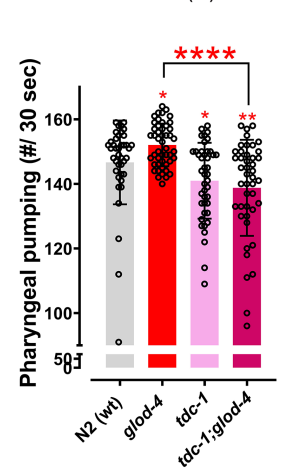
Figure 3. Worms carrying the tdc-1 mutation displayed a suppressed feeding rate both as single and double mutants.
Continuing down the path, a question naturally presents itself, “How does tyramine regulate the increase in feeding induced by MG-H1?” The researcher discovered that treating the wild type animals with tyramine resulted in a suppression of feeding. However, the reverse effect was discovered in trials with glod-4 double mutants, which restored the feeding rate to the elevated level observed in glod-4 single mutants (Figure 4). In conclusion, tyramine is capable of suppressing feeding in wild type animals and increasing PPR in the glod-4 mutant background. Hence, tyramine signaling is necessary for mediating the glod-4 mutant-dependent increase in feeding behavior.
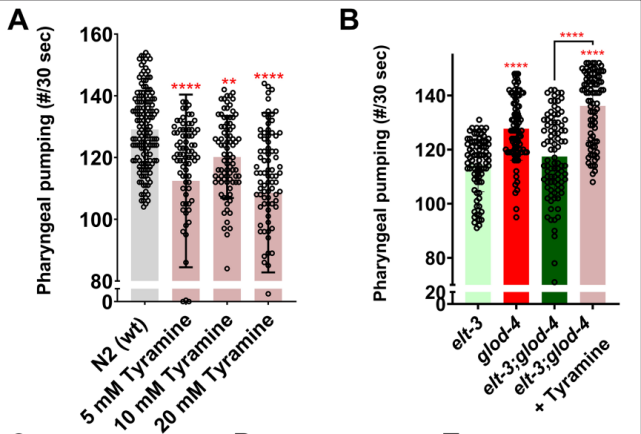
Figure 4. The effect of exogenous tyramine supplement on wild type and glod-4 double mutants.
Previous studies have established that the accumulation of α-DCs in glod-4 mutants leads to a pathogenic phenotype, including neurodegeneration and a shortened lifespan. This study demonstrates that the accumulation of MG-H1 significantly reduces the lifespan of wild type nematodes. We mentioned above that tyramine signaling is necessary for regulating the glod-4 mutant-dependent increase in feeding. When we blocked the tyramine signaling pathways, we observed a decrease in the pathogenic phenotype associated with the mutant worms. It was reported that the lifespan of glod-4 significantly increased when tyramine signaling was inhibited in the tdc-1;glod-4 double mutation. A similar effect was observed for double mutations in the tyramine receptor genes, tyra-2 and ser-2 (Figure 5).
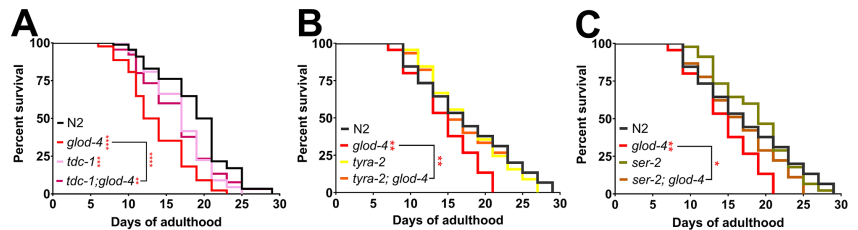
Figure 5. Lifespan assay of wild type and several glod-4 double mutants.
The connection between obesity and chronic diseases is indeed complex and intertwined. Living a sedentary lifestyle and consuming an excessive amount of food and drinks can contribute to weight gain and increase the risk of developing chronic diseases. If one indulges in a lavish lifestyle, consuming and imbibing to their heart's content, it is difficult to avoid the consequences unharmed. The accumulation of AGEs resulting from the Maillard reaction can potentially lead to the development of unhealthy eating habits. This creates a vicious cycle where the consumption of unhealthy foods leads to the formation of AGEs, which in turn may contribute to the development of further unhealthy eating habits. It is essential that we understand the impact of our lifestyle choices on our health and take steps to address them. This may involve adopting a balanced and nutritious diet, engaging in regular physical activity, and seeking professional guidance when necessary.
Reference:
Muniesh Muthaiyan Shanmugam, Jyotiska Chaudhuri, Durai Sellegounder, Amit Kumar Sahu, Sanjib Guha, Manish Chamoli, Brian Hodge, Neelanjan Bose, Charis Roberts, Dominique O Farrera, Gordon Lithgow, Richmond Sarpong, James J Galligan, Pankaj Kapahi (2023) Methylglyoxal-derived hydroimidazolone, MG-H1, increases food intake by altering tyramine signaling via the GATA transcription factor ELT-3 in Caenorhabditis elegans eLife 12:e82446
https://doi.org/10.7554/eLife.82446







