Cholesterol Emerges as a Master Switch for Growth Restart in C. elegans
2024-03-18 17:36
The microscopic worm Caenorhabditis elegans has become a powerful model organism for understanding developmental biology and aging. A noteworthy characteristic exhibited by this nematode is the dauer stage, a period of developmental arrest triggered by harsh environmental conditions like limited food or overcrowding. During diapause, the worm enters a state of suspended development, thereby reducing its metabolic rate and conserving resources. When conditions improve, C. elegans remarkably restarts growth and resumes its life cycle. A recent study published in Communications Biology by Schmeisser et al. (2024) sheds light on a surprising player in this growth resumption process, cholesterol. Their work reveals that the mobilization of cholesterol stores triggers the transition from dauer diapause to active growth through its influence on steroid hormone production and mTOR signaling.

The dauer stage is characterized by a suite of physiological adaptations, one alteration occurring in the gut, which transforms into a storage organ for cholesterol. When C. elegans enters dauer, cholesterol is bound to SCL-12 and SCL-13 and is transported into the intestinal lumen and stored there throughout the duration of the dauer stage.(Figure 1).This raises an interesting question: why sequester cholesterol during a period of developmental arrest? The authors propose that this serves a protective purpose. By binding to these proteins, cholesterol is likely rendered less susceptible to oxidation, a process that can damage cellular components. Additionally, storing cholesterol in the gut lumen keeps it away from essential cellular processes, further promoting metabolic quiescence.
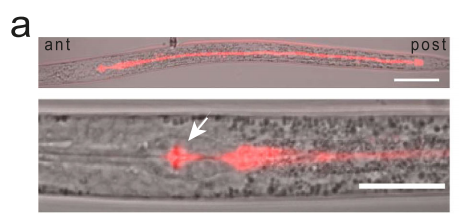
Figure 1. SCL-12::mScarlet reporter dauer larva.
When environmental conditions become favorable, C. elegans initiates a series of events to resume development and growth. A key aspect of this process involves the mobilization of the sequestered cholesterol stores. The study demonstrates that upon dauer exit, the protein-bound cholesterol complexes are transported to the lysosomes, where the proteins are presumably degraded and cholesterol is released. Within the intestinal cells, lysosomes, which are membrane-bound compartments responsible for cellular waste disposal, break down the SCL-12 and SCL-13 proteins(Figure 2). This liberation of free cholesterol enables it to exert its influence on downstream signaling pathways.
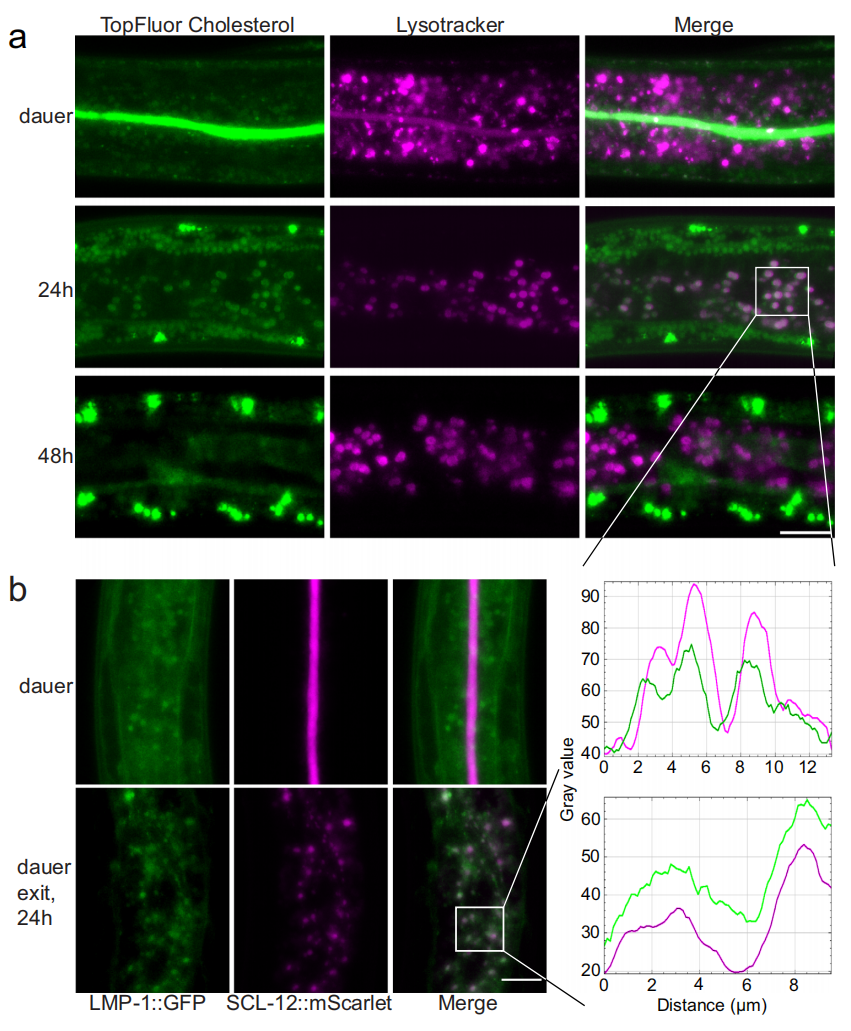
Figure 2. Cholesterol is transported from the gut lumen to intestinal lysosomes with SCL-12 and then further to the epidermal region.
The freed cholesterol acts as a potent signal for restarting growth through two main mechanisms:
1. Steroid hormone production: Cholesterol serves as a precursor molecule for the synthesis of various steroid hormones, including dafachronic acids. The research team found that upon dauer exit, the released cholesterol is used by intestinal cells to produce these hormones. Dafachronic acids are known to promote longevity and stress resistance in C. elegans. Interestingly, the study demonstrates that they also play a role in stimulating growth after dauer(Figure 3).
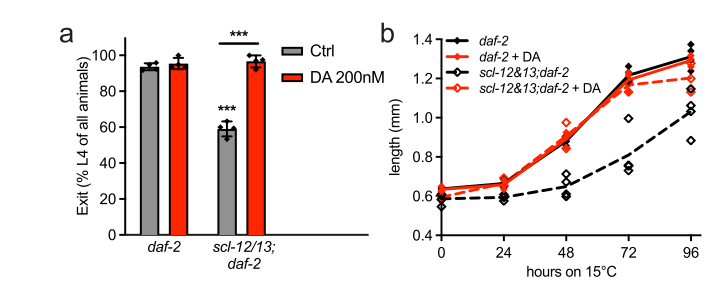
Figure 3. 200 nM DA fully restored recovery rate and growth during dauer exit at 15 °C
2. mTORC1 signaling activation: Cholesterol also appears to influence a critical growth regulatory pathway known as mTOR signaling. Utilizing a conditional knockout of a protein essential for mTORC1 function (DAF-15), the researchers demonstrate that cholesterol indeed activates mTORC1 in the intestinal cells after dauer exit(Figure 4). mTORC1 is a master regulator of cell growth and metabolism, and its activation promotes protein synthesis, a fundamental process for building new tissues and growth.
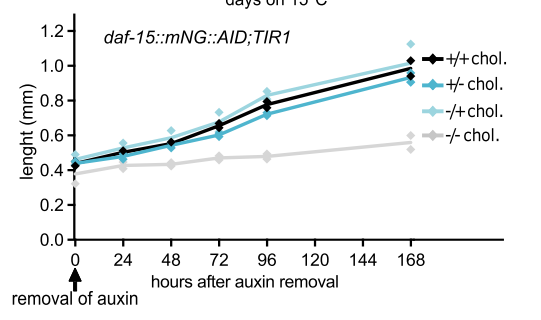
Figure 4. worms treated with cholesterol resumed growth after exiting the auxin-induced arrest while the ones without cholesterol didn’t.
The study by Schmeisser et al. (2024) establishes a fascinating link between cholesterol mobilization, steroid hormone production, and mTOR signaling in the context of dauer exit in C. elegans. Their findings suggest that cholesterol acts as a metabolic switch, signaling the transition from developmental arrest to active growth. We can extract the following highlights:
-. During dauer, cholesterol is sequestered in the gut lumen bound to specific proteins, likely for protection and to maintain metabolic quiescence.
-. Upon favorable environmental cues, cholesterol is released from its protein carriers through endocytosis by intestinal cells.
-. Freed cholesterol serves as a precursor for the synthesis of dafachronic acids, hormones that promote growth and development.
-. Cholesterol also activates mTORC1 signaling within the intestinal cells, further stimulating protein synthesis and growth.
This research presents promising avenues for future investigation. A crucial inquiry lies in elucidating the mechanism by which cholesterol initiates downstream signaling cascades. Does it engage in direct interactions with specific protein receptors or enzymes? Additionally, researchers could explore the precise roles of dafachronic acids in the growth resumption process. Beyond C. elegans, the findings might have broader implications for understanding how organisms regulate growth and developmental transitions. The study highlights the potential of cholesterol as a signaling molecule beyond its well-established role in lipid metabolism. It also underscores the importance of studying how organisms switch between active growth and dormant states, with potential applications in areas like regenerative medicine and aging research.
Reference:
Schmeisser K, Kaptan D, Raghuraman BK, Shevchenko A, Rodenfels J, Penkov S, Kurzchalia TV. Mobilization of cholesterol induces the transition from quiescence to growth in Caenorhabditis elegans through steroid hormone and mTOR signaling. Commun Biol. 2024 Jan 24;7(1):121. doi: 10.1038/s42003-024-05804-7. PMID: 38267699; PMCID: PMC10808130.







