The Astonishing Discovery of Glutaminase: The Unsung Hero Behind Sperm Function
2025-05-13 11:21
Keywords: glutaminase, sperm function, cellular redox homeostasis, male infertility
Introduction
Decreased sperm function is one of the major causes of male infertility. In humans, abnormalities in sperm motility, morphology, and quantity can all affect conception. However, many aspects of the regulatory mechanisms of sperm function remain unknown. Glutaminase plays a key role in cellular metabolism, responsible for converting glutamine into glutamate, which is involved in energy metabolism and biosynthesis. Although its functions in cancer cells and neurons have been widely studied, its role in sperm function is still unclear. A recent study published in iScience used Caenorhabditis elegans (C. elegans) to explore the impact of glutaminase ortholog loss on sperm function, providing new clues for mammalian reproductive research.
Construction of SunyBiotech
SunyBiotech constructed key C. elegans strains, providing a foundation for elucidating the crucial role of glutaminase in maintaining sperm function:
PHX1405: glna-1(syb1405)
PHX1406: glna-2(syb1406)
PHX1403: glna-3(syb1403)
PHX4148: gfp::glna-1
PHX4077: glna-2::gfp
PHX3987: glna-3::gfp
1. Glutaminase deficiency affects fertility
Using CRISPR/Cas9 gene-editing technology, the three glutaminase ortholog genes in C. elegans (glna-1, glna-2, and glna-3) were knocked out to generate mutant strains. The study found that mutant worms had significantly reduced self-fertilization offspring and increased numbers of unfertilized oocytes (Fig. 1A-B). The sperm of mutant male worms were capable of fertilization, but their competitive ability with hermaphrodite sperm was compromised (Fig. 1C). Moreover, mutant worms had reduced spermatids numbers, smaller diameters, and decreased motility (Fig. 1D-1F). These findings indicate that the absence of glna genes disrupts sperm development in C. elegans, leading to decreased sperm function.
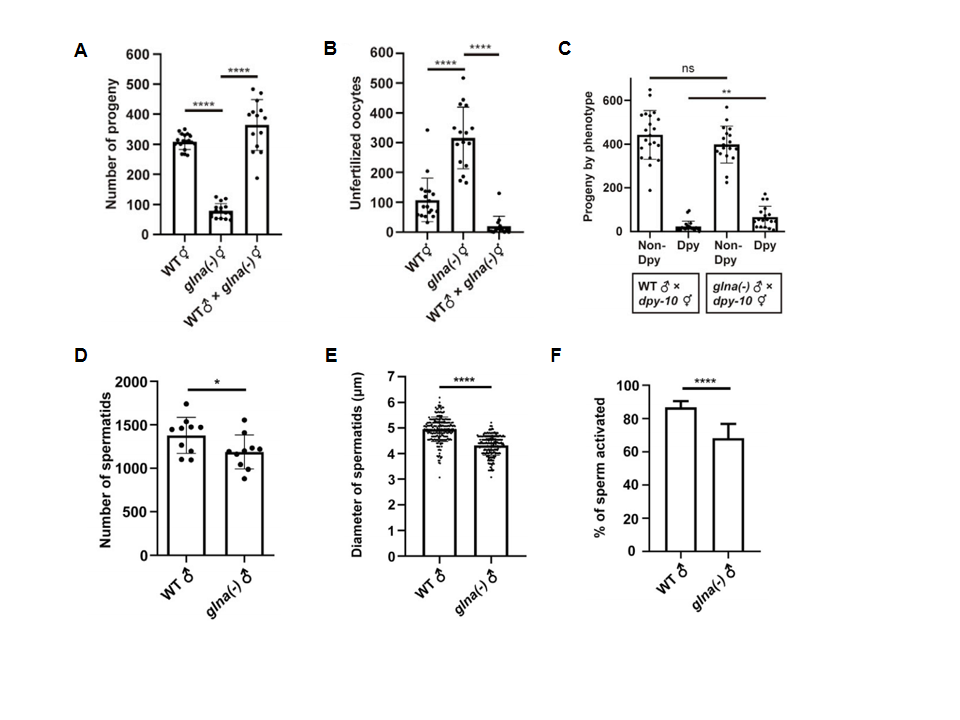
Figure 1. Glutaminase deficiency affects fertility
2. Germline glna(+) is required for sperm function
GFP fusion proteins were used to indicate the expression locations of glna in worms (Fig. 2A). It was found that glna-1 was expressed in mitotic germline precursors and developing spermatocytes, while glna-3 was expressed in the spermatids (Fig. 2B-2J). After knocking down glna-3 in spermatids via RNAi, significant reductions in sperm cell size and activation were observed, along with a decrease in self-fertilization offspring (Fig. 2K-2M). This demonstrates that the activity of glna in germline cells is crucial for promoting sperm function in C. elegans.
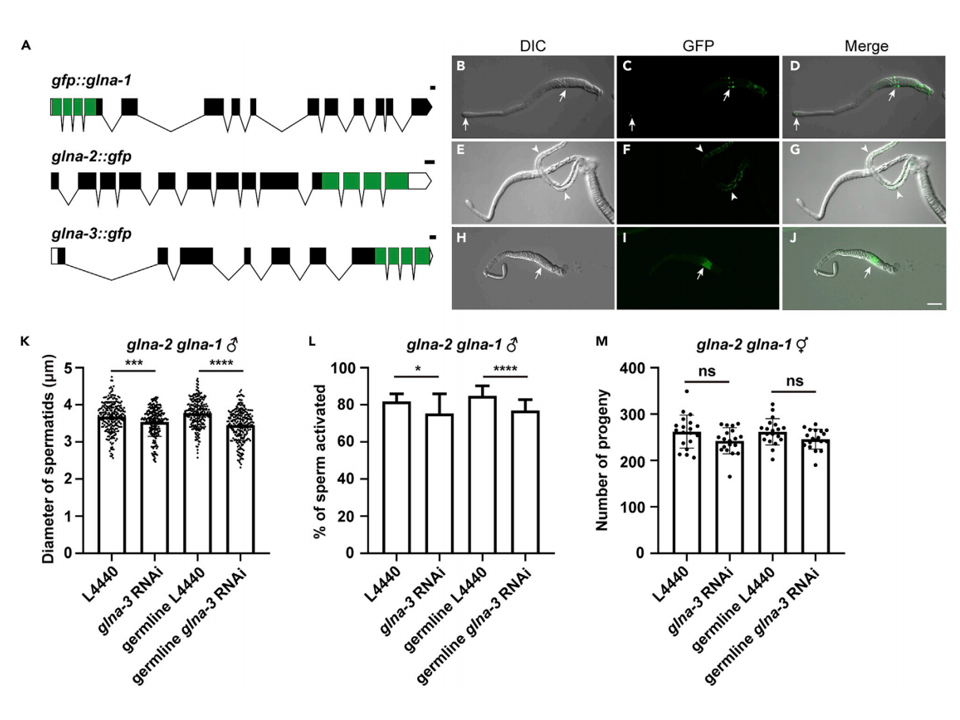
Figure 2. Germline glna(+) is required for sperm function
3. Glna promotes sperm function by maintaining cellular redox homeostasis
Glutathione is a cellular antioxidant that neutralizes reactive oxygen species (ROS), and glutaminase is essential for the synthesis of glutathione. The study found that ROS levels were elevated in glna(-) mutant strains (Fig. 3A), but adding the antioxidant NAC significantly restored sperm size and motility (Fig. 3B and 3C). This indicates that glutaminase promotes sperm function by maintaining cellular redox balance, which is crucial for sperm motility, morphology, and fertilization ability.
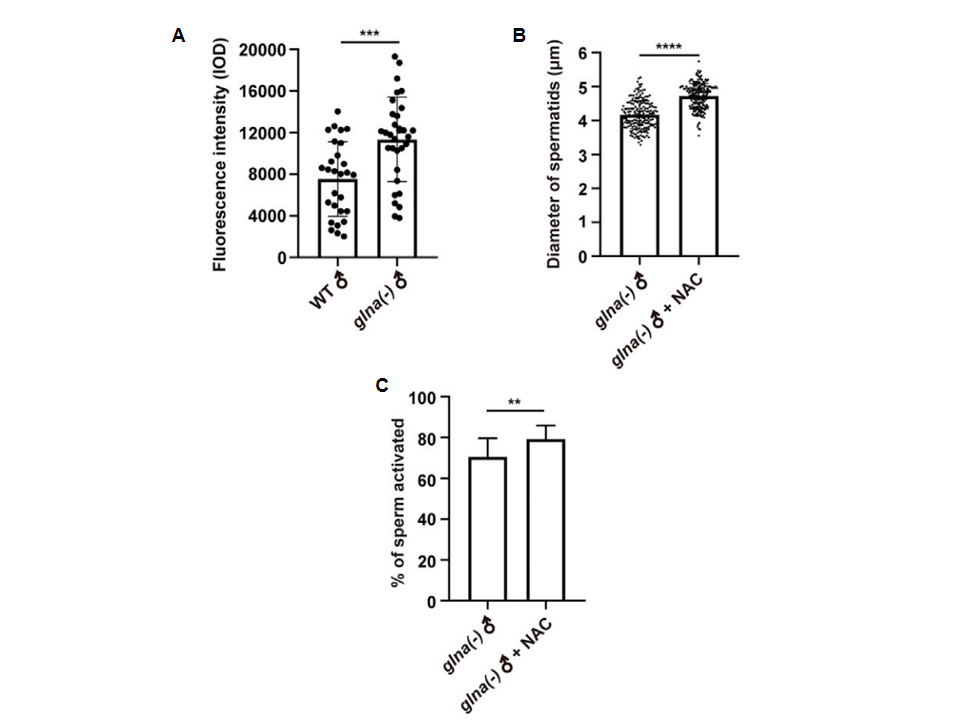
Figure 3. Glna promotes sperm function by maintaining cellular redox homeostasis
Conclusion
This study revealed the significant impact of glutaminase orthologs on sperm function by knocking them out in C. elegans, emphasizing their importance in maintaining cellular redox balance. Since the function of glutaminase may be conserved across different species, this discovery not only provides a new perspective for understanding the molecular mechanisms of sperm function but also offers a potential target for developing new strategies to treat human male infertility. Future research can further explore the role of glutaminase in other reproductive processes, as well as its feasibility and safety as a therapeutic target.
References
Liang Q, Yang H, Zhang Z, et al. Loss of mammalian glutaminase orthologs impairs sperm function in Caenorhabditis elegans. iScience. 2023 Feb 15;26(3):106206.





