Reproductive Regulation in Caenorhabditis elegans: A New perspective on Mitochondrial Stress Response
2024-11-11 16:27
Keywords: mitochondrial stress response, unfolded protein response, germline, proteostasis, reproduction
Introduction
Protein homeostasis (proteostasis) is essential for maintaining cellular and organismal balance, and its disruption is a defining characteristic of aging. The mitochondrial unfolded protein response (UPRmt) is a key mechanism in maintaining proteostasis. One key player in this system is the mitochondrial UPRmt, a mechanism that helps cells cope with mitochondrial stress. This response is initiated in a non-cell autonomous fashion across various tissues to manage mitochondrial stress, yet the precise mechanisms by which inter-tissue signaling triggers UPRmt remain elusive.
In this study, the authors explore the exchange of information between the germline and somatic cells, uncovering a critical role for a fully formed and reproductively active germline in the systemic activation of the mitochondrial stress response in multicellular organisms. These new findings uncover insights that could advance the understanding of the reproductive regulation of UPRmt.
1. Somatic UPRmt activation requires intact germline
In this study, glp-1 mutants were utilized to investigate germline attenuation, a process in which germline stem cells (GSCs) are progressively depleted at 25℃. UPRmt activation was monitored using Phsp-6::GFP, a reporter that fluoresces under mitochondrial stress. Prior to depletion of GSCs, glp-1(e2141ts) and glp-1(e2144ts) induced Phsp-6GFP with a capacity comparable to that of wild type (Fig 1A). However, in the absence of GSCs, neither of them was able to induce Phsp-6GFP (Fig 1B), similar to atfs-1(tm4525), which was unresponsive to UPRmt.
Further analysis using mes-1(bn7ts) mutants showed that UPRmt activation depends on a fully functional germline. Specifically, it was observed that the sterile variant of the mes-1(bn7ts) mutant failed to induce phsp-6 GFP expression following stress treatment(Figure 1D), whereas the fertile counterpart exhibited the opposite response (Figure 1C).
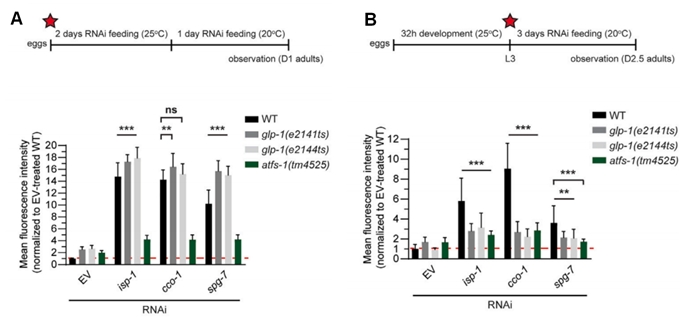
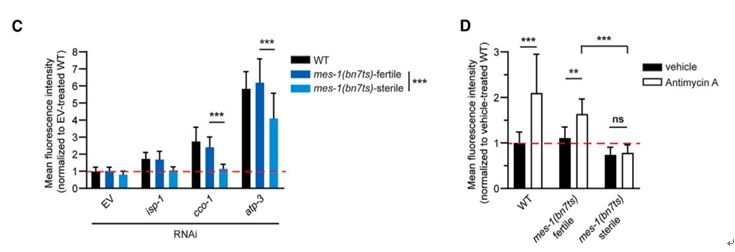
Figure 1. Somatic UPRmt activation requires intact germline
2. Somatic UPRmt is associated with oocytes and active reproduction
The study also explored the link between active reproduction and UPRmt. Maturation and development of the oocyte is essential for active reproduction. By investigating mutants defective in either sperm or oocyte production, the authors showed that reproductive activity is essential for maintaining somatic UPRmt induction. Specifically, sperm-and oocyte-deficient mutants, such as fem-1(hc17ts) and fem-3(q20gof), displayed reduced UPRmt activation, but mating with wild-type males partially restored this capacity (Figure 2A).
Moreover, genetic repression of cco-1 and atp-3 successfully induced Phsp-6 GFP expression in hermaphrodites (with XX sex chromosomes), but not in males (with XO sex chromosomes) (Figures 2B and 2C), suggesting that UPRmt exhibits sexual dimorphism in stress response.
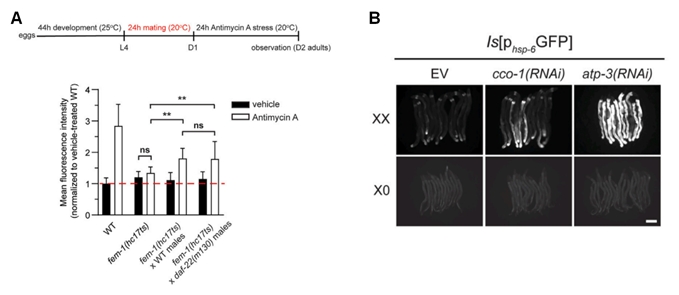
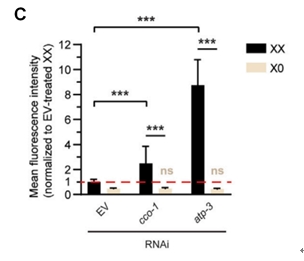
Figure 2. Reproduction is coupled with somatic UPRmt
3.The interplay between germline and somatic UPRmt
Finally, the authors investigated whether mitochondrial stress in the germline could induce UPRmt in somatic tissues. Using tissue-specific RNAi mutants, they found that mitochondrial stress in the germline alone could trigger UPRmt in somatic cells, although the effect was weaker compared to systemic stress. It is related to the mechanism and strength of the signaling. Germline stress is specific and activates UPRmt through non-cell-autonomous mechanisms. Germline-specific mitochondrial stress induced a weaker UPRmt response in somatic cells (Figure 3), showing that germline-to-somatic communication plays a role, but full UPRmt activation requires broader tissue involvement. In addition to the germline, tissues such as the nervous system and gut are also involved in the process of UPRmt activation. These cross-tissue signaling pathways act synergistically to regulate the mitochondrial stress response.
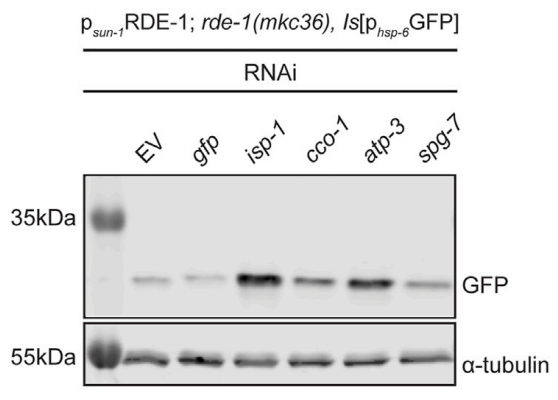
Figure 3. Mitochondrial stress in the germline induced somatic UPRmt
Conclusion
The UPRmt is a critical mechanism that enables organisms to recover from mitochondrial stress, and there is a significant association between reproductive signaling and the activation of UPRmt. Efficient induction of somatic UPRmt requires an intact and active germline and it exhibits sexual dimorphism. Mitochondrial stress in the germline can activate somatic UPRmt, although the effect is weaker compared to systemic stress. The findings open up new avenues for future research, which may explore whether fertility alone is sufficient to sustain the full activity of UPRmt. Such investigations could provide deeper insights into the complex mechanisms that underpin the relationship between reproductive health and cellular stress responses.
References:
Nikolaos Charmpilas, Aggeliki Sotiriou, Konstantinos Axarlis et al. Reproductive regulation of the mitochondrial stress response in Caenorhabditis elegans. Cell Reports, 2024 Jun 25;43(6):114336. doi: 10.1016/j.celrep.2024.114336.







