Post-Transcriptional Repression of CFP-1: Expanding LIN-41’s Regulatory Toolkit
2025-08-01 18:56
Keywords
LIN-41/TRIM71, CFP-1 repression, Germline regulation, CCR4-NOT deadenylation, Oocyte-to-embryo transition (OET)
Contribution of SunyBiotech
SunyBiotech is very honored to provide strain construction services for this study. Strains sybIs5469[(Pmex-5::gfp::H2B::PEST::cfp-1 3′UTR) II];unc-119(ed3) III, sybIs5817[(Pmex-5::gfp::H2B::PEST::cfp-1 3′UTR(LRE mutant)) II];unc-119(ed3) III and cfp-1(syb3876[cfp-1::mCherry::myc])IV were created for this work by SunyBiotech Corporation.
Introduction
RNA-binding proteins (RBPs) recognize RNA sequences or structural features through diverse RNA binding domains (RBDs), participate in metabolic processes such as mRNA splicing, stability regulation, and translation control, and thereby coordinate gene expression. The NHL domain, named after the Caenorhabditis elegans (C. elegans) proteins NCL-1, HT2A and LIN-41, is an RBD found in the conserved TRIM-NHL family of RBPs. Among these, LIN-41 (TRIM71 in mammals) is best known as a player in the so-called heterochronic pathway that regulates developmental transitions in the soma. Additionally, LIN-41 functions in the germline, where it controls various aspects of the oocyte-to-embryo transition (OET), including the meiotic progression and reprograming into pluripotency. While the function of LIN-41 in the heterochronic pathway is linked to specific mRNAs, the identity of germline targets relevant for the various aspects of OET is less clear.
A study published in Nucleic Acids Research uncovers a new layer of LIN-41’s activity: its ability to repress a key chromatin modifier, CFP-1, in the germline, shedding light on how cells reprogram their identity during early development.
Key findings:
1: Lin-41: A multitool regulator
In C. elegans, LIN-41 operates in two distinct contexts. In the soma, it orchestrates developmental transitions by binding specific mRNAs via short RNA structures called LIN-41 Recognition Elements (LREs). Depending on where LREs are located (in the 5′ or 3′ untranslated regions of mRNAs), LIN-41 either degrades the mRNA or halts its translation. In the germline, LIN-41 takes on a different mission: ensuring oocytes mature properly and transition into embryos. Without LIN-41, germ cells lose their identity, abort meiosis, and even form teratoma-like masses—suggesting LIN-41 prevents premature activation of embryonic genes. The research indicates that LIN-41 appears to be capable of translationally repressing mRNA via LREs also in the germline, through a mechanism that may involve cytoplasmic deadenylation.
2: cfp-1: A new target in the germline
To identify LIN-41’s germline targets, the authors combined RNA immunoprecipitation (RIP‑seq) with computational searches for LRE motifs. This screen highlighted cfp‑1, encoding a conserved H3K4 methyltransferase adaptor, as a strong candidate. CFP-1 is a conserved chromatin modifier, part of complexes that mark active genes (via H3K4 trimethylation) and interact with histone deacetylases. Its role in early development made it a compelling target: if LIN-41 represses CFP-1, it could delay embryonic gene activation until the oocyte is ready to become an embryo. Using GFP reporters fused to the cfp‑1 3′ UTR, they demonstrated that in wild-type worms, GFP was silenced in oocytes (where LIN-41 is active) but turned on when LIN-41 was depleted—confirming LIN-41 represses cfp-1. Crucially, mutating the LREs in the cfp‑1 3′ UTR abolished repression, proving direct, element‑dependent targeting.
Mechanistically, LIN‑41 recruits the CCR4‑NOT deadenylase complex to shorten cfp‑1’s poly(A) tail and block translation. Knockdown of CCR4‑NOT subunits such as CCF‑1 or NOT‑1 lifted repression of the cfp‑1 reporter (Figure 1), whereas somatic LIN‑41 targets remain CCR4‑NOT independent. This finding underscores LIN‑41’s adaptability: it utilizes distinct effector pathways in different tissues to achieve context‑specific regulation.
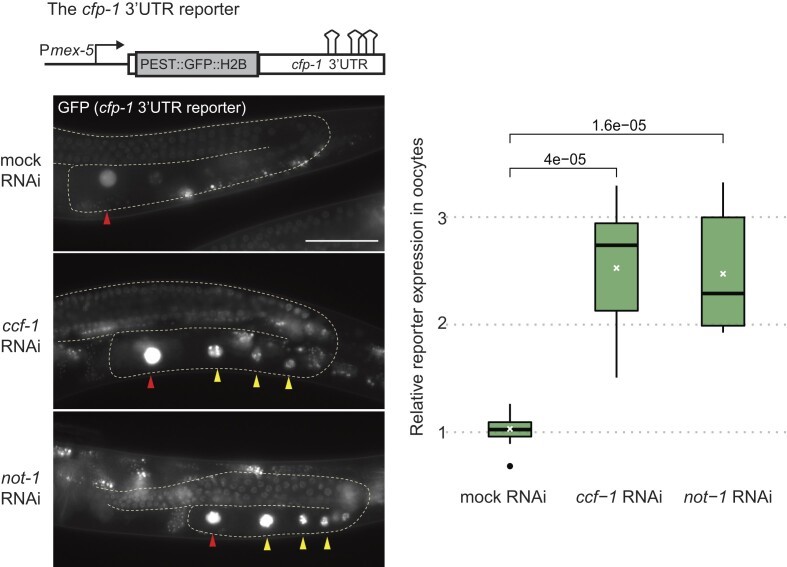
Figure 1. The CCR4–NOT deadenylase complex is required for the translational repression of cfp-1.
The consequences of losing LIN-41 are stark: germ cells activate embryonic genes prematurely. The team showed CFP-1 is role in depositing H3K4me3 marks makes it a potent driver of transcriptional activation. Genes representing EEGs (vet-4, vet-6), somatic lineage specific genes (hlh-1, unc-120, end-1) and hox genes (mab-5, ceh-13), were all upregulated in lin-41(rrr3) mutants compared with wild-type (Figure 2). However, RNAi-mediated depletion of CFP-1 strongly reduced the levels of most tested transcripts (Figure 2). Thus, LIN‑41’s repression of cfp‑1 acts as a molecular “gatekeeper”, delaying chromatin changes until oocytes are poised for the OET.
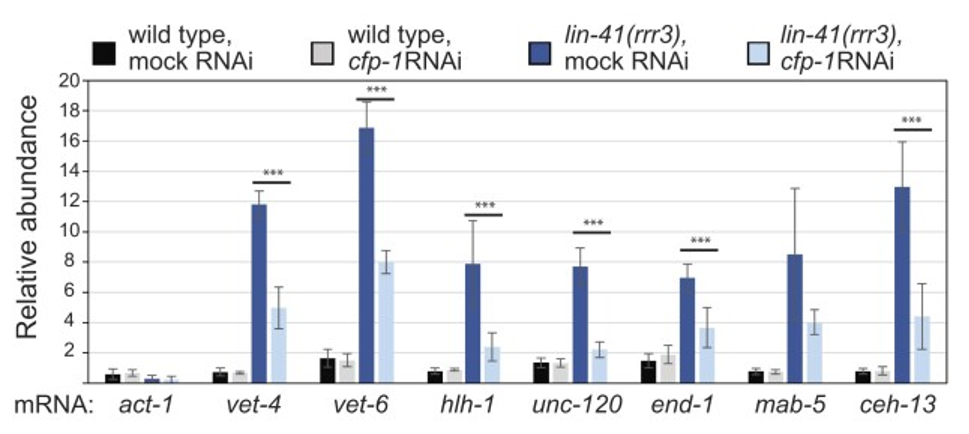
Figure 2. CFP-1 is required for the expression of early embryonic genes in wild-type embryos and lin-41(-) mutant gonads. RT-qPCR analysis comparing the abundance of selected mRNAs in the gonads dissected from animals of the indicated genotypes.
3. Evolutionary conservation and broader implications
Intriguingly, mammalian CFP1 is similarly downregulated in maturing oocytes and early embryos, suggesting that post‑transcriptional silencing of chromatin regulators may be a universal strategy to synchronize epigenetic reprogramming with developmental timing. In C. elegans, LIN-41’s toolkit now includes both indirect (via OMA-1/2) and direct (via LREs) mRNA regulation, each targeting distinct genes for specific roles. For CFP-1, direct LRE binding and CCR4-NOT recruitment ensure precise control, critical for balancing germ cell identity and embryonic potential.
Conclusion
This study paints a richer picture of LIN-41’s versatility, showing it adapts its mechanisms to suit the needs of different tissues. By uncovering CFP-1 as a key target in the germline, it also bridges post-transcriptional regulation with epigenetic reprogramming—two layers of control that must align for life to begin. As we learn more about LIN-41 and its mammalian homologs, we edge closer to understanding how cells navigate one of life’s most dramatic transitions: from a single oocyte to a multicellular embryo.
Reference
Kumari P, Thuestad LH, Ciosk R. Post-transcriptional repression of CFP-1 expands the regulatory repertoire of LIN-41/TRIM71. Nucleic Acids Res. 2023;51(19):10668-10680.
doi:10.1093/nar/gkad729




