Exploring the Intricacies of Protein Fatty Acylation in Caenorhabditis elegans: Amino Acid and Protein Specificity
2025-08-28 10:11
Keywords: protein lipidation, click chemistry, proteomics, branched-chain fatty acids, Caenorhabditis elegans
Introduction
In the fascinating realm of molecular biology, the intricate mechanisms that govern protein function and regulation continue to captivate researchers. One such mechanism that has garnered significant attention is protein fatty acylation. This post-translational modification, involving the covalent attachment of fatty acids to proteins, plays a crucial role in various cellular processes, including membrane association, protein-protein interactions, and signal transduction. However, the specificity of amino acids and proteins involved in this process remains a complex and intriguing puzzle. An article published in PNAS has delved deeply into the phenomenon of protein fatty acylation in the model organism Caenorhabditis elegans (C. elegans), attempting to uncover the secrets behind the unique amino acid and protein specificity that drives this important biological process. By dissecting the underlying mechanisms and revealing the key players, this research provides new insights into the significance of protein fatty acylation in the field of cell biology and beyond.
Construction of SunyBiotech
SunyBiotech constructed two mutant strains for validating the S-acylation sites of the ZIG-1 protein and their functional relevance:
PHX8149 zig-1(syb8149)
PHX8254 zig-1(syb8149 syb8254)
1. Amino acid specificity
In C. elegans, the pattern of fatty acylation displays pronounced preferences for particular amino-acid residues. S-acylation of cysteine occured predominantly with 15-methyl-hexadecanoic acid (isoC17:0), a monomethyl-branched-chain fatty acid (mmBCFA) generated from endogenous leucine metabolism (Fig. 1A and 1B). By contrast, the N-terminal glycine was almost exclusively myristoylated with a straight-chain myristic acid (Fig. 1C). Lysine residues were preferentially acylated by two distinct monomethyl-branched-chain fatty acids (Fig. 1D), whereas serine was modified by a broad spectrum of fatty acids, including eicosapentaenoic acid (Fig. 1E). Collectively, these results reveal a highly specific acyl-fatty-acid signature profile for each amino-acid residue.
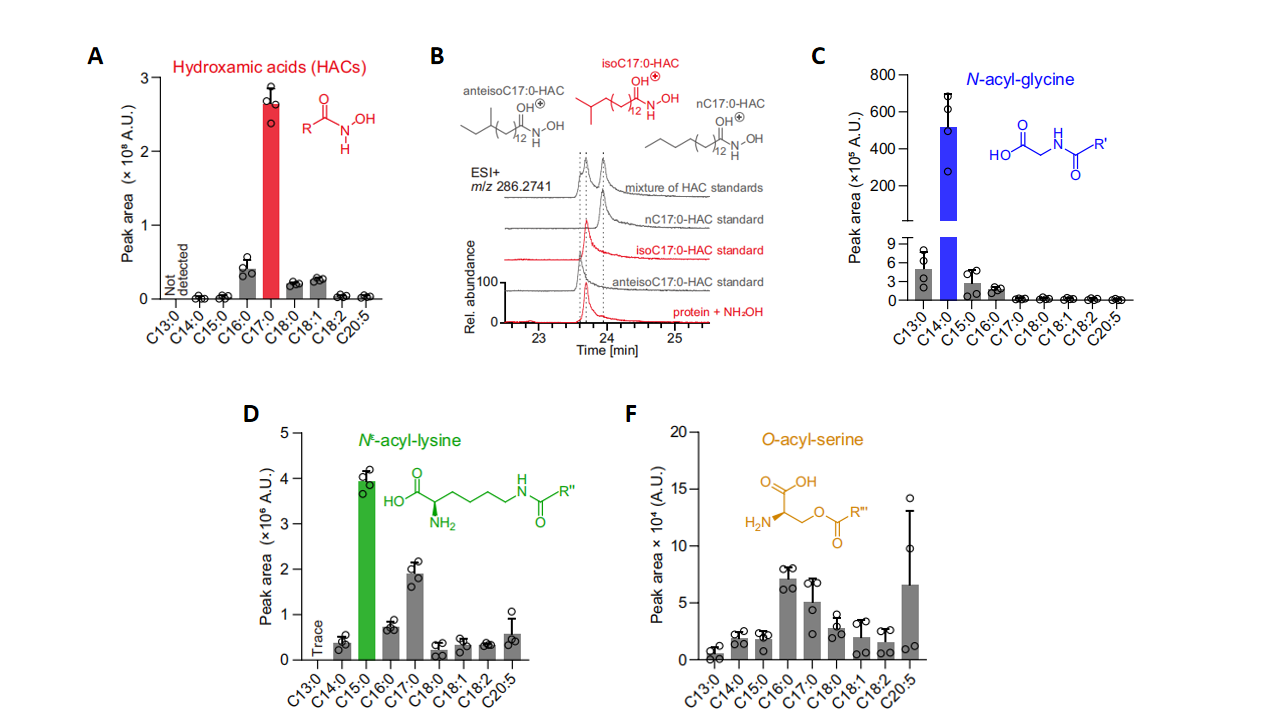
Figure 1. Amino acid specificity
2. Protein specificity
Using click chemistry that coupled alkyne-based probes, researchers performed a global survey of branched- and straight-chain fatty acids and identified 1013 S-acylated proteins and 510 hydroxylamine-resistant N- or O-acylated proteins (Fig. 2A and 2B). Notably, certain S-acylated proteins were labeled almost exclusively by either the branched- or the straight-chain probe, indicating acylation specificity at the protein level. For example, the S-acyltransferase DHHC-10 was shown to exhibit selectivity for particular fatty acids (Fig. 2C and 2D). This protein-level specificity further indicates that fatty acylation depends not only on the nature of the amino acid residues but also on the overall structure and microenvironment of the protein.
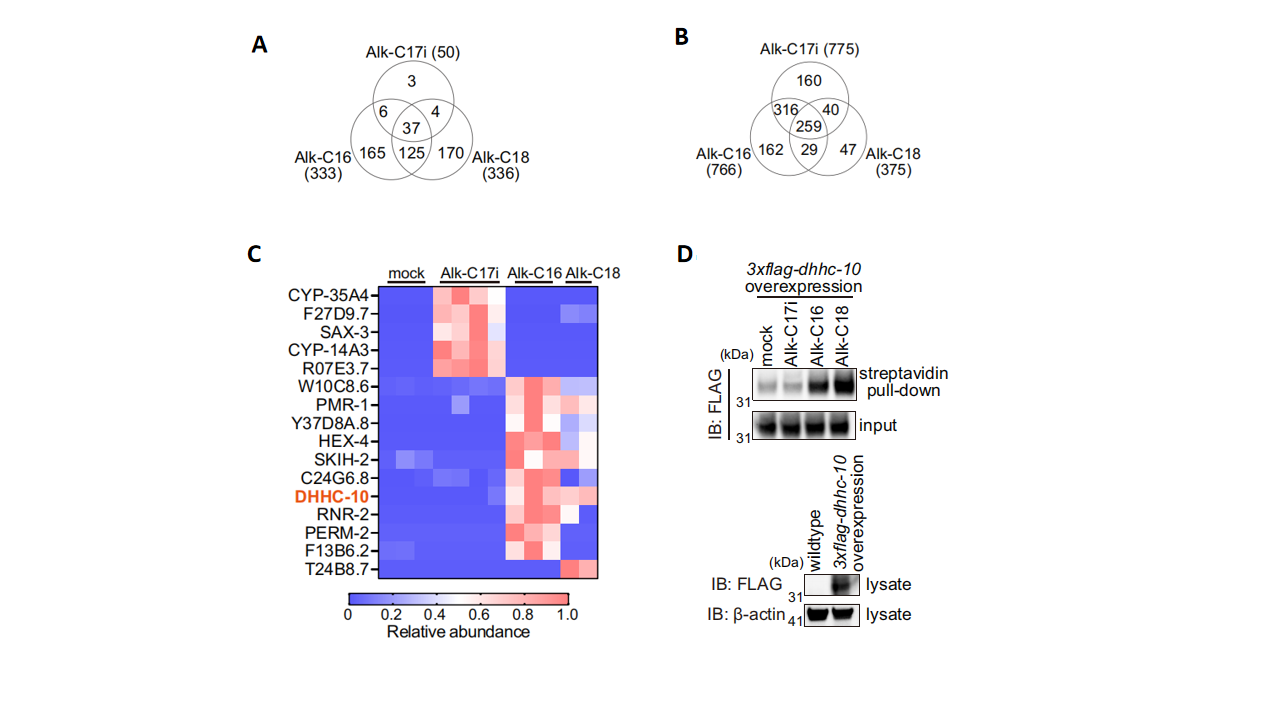
Figure 2. Protein specificity
3. Conservation of acylation sites
Homology searches for the identified acylated proteins revealed a high degree of conservation of acylation site patterns across metazoa(Fig. 3). This suggests that the mechanisms underlying protein fatty acylation in C. elegans may have broader relevance to other organisms, including humans. Understanding these specific fatty-acylation patterns could provide valuable insights into diverse biological processes and into diseases linked to protein fatty acylation.
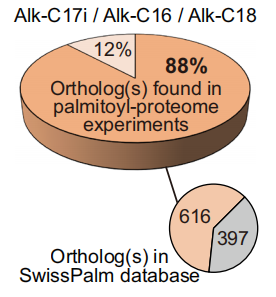
Figure 3. Conservation of acylation sites
Conclusion
Investigations of protein fatty acylation in C. elegans have uncovered pronounced specificity at the levels of both amino acids and individual proteins. This specificity integrates distinct branches of lipid metabolism, highlighting the intricate interplay between protein modification and lipid biosynthesis. Future research in this area is expected to deepen our understanding of fundamental biological processes and to aid in the development of therapeutic strategies for diseases associated with protein fatty acylation.
References
Zhang B, Yu Y, Fox BW, Liu Y, Thirumalaikumar VP, Skirycz A, Lin H, Schroeder FC. Amino acid and protein specificity of protein fatty acylation in C. elegans. Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2307515121.





