Decoding lifespan secrets: the gonad removal in Caenorhabditis elegans
2024-10-16 18:14
Keywords: diapause, evolution of aging, lipids, germline, reproduction
Introduction:
Aging is a complex process characterized by a decline in physiological functions. The physiological mechanisms of aging have been continuously explored using C. elegans to unravel the code of longevity. Current research on the theory of aging aging theory focuses primarily on insulinand germline signaling pathways, with the latter being relatively understudied.
Studies have shown that removing the reproductive precursor cells (Z2 and Z3) by laser cell ablation in the first larval stage of C. elegans extends the lifespan.Similarly, in the wild-type C. elegans adult, lifespan is significantly extended in glp-1 loss-of-function nematodes, which mimic the Z2/Z3-ablated animals because both lack proliferating and undifferentiated germ cells. Like glp-1, the glp-4 (loss-of function temperature-sensitive allele) nematodes had an extended lifespan, but when grown on dead E. coli. At restrictive temperatures, mes-1 (temperature-sensitive) mutants do not develop the germline precursors Z2 and Z3, resulting in lifespan extension. After germline removal, transcriptional regulators such as DAF-16 and DAF-12 drive significant changes in lipid metabolism, which are closely linked to lifespan extension.When starvation during late larval stages induces quiescence in germline stem cells, adult nematodes enter reproductive diapause(ARD), also leading to an extended lifespan.Consequently, the gonad has emerged as a key organ in understanding aging in C. elegans.
This review aims to address the mechanisms behind the increased lifespan of mutants lacking proliferating germ cells, connecting these findings with recent theories of aging, identifying gaps in the literature, and suggesting potential future research directions.These insights may further our understanding of lifespan regulation in C. elegans.
The review:key conclusions
1. Changes in transcriptional regulation and lipid metabolism after germline removal
The glp-1 gene, a member of the Notch receptor family, plays a crucial role in the regulation of germline cell proliferation. In germline-deficient nematodes, transcriptional regulators like DAF-12 and DAF-16, which promote longevity, undergo significant alterations.. DAF-12 facilitates the translocation of DAF-16 from the cytoplasm to the nucleus and activates the fatty acid reductase fard-1, a gene required for lifespan extension in animals lacking germline. DAF-16 controls genes involved in lipid metabolism, such as lipl-1, lipl-2, and fat-5, which play key roles in fat processing and energy storage.
Additionally, in addition, LIPL-4, an enzyme regulated by DAF-16, activates NHR-49 and NHR-80, which play critical roles in mitochondrial β-oxidation and longevity. NHR-49 promotes fat synthesis, while NHR-80 helps regulate lipid metabolism by controlling the production of desaturases.
In glp-1 mutants, DAF-16 activity is uniquely regulated by proteins like MBK-1, TCER-1, and KIL-1, which are not involved in other long-lived pathways such as the insulin signaling pathway. Additionally, following germline removal, lipid metabolism in the gut is regulated by the transcription factor SKN-1. In glp-1 mutants, SKN-1 works with NHR-49 to increase the expression of genes that boost mitochondrial β-oxidation, a process that increases energy production and reduces fat storage. Lysosomal lipase LIPL-4 produces oleoylethanolamide (OEA), a monounsaturated fatty acid that binds to the lipid chaperone LBP-8, promoting the nuclear translocation of NHR-80 and NHR-49.
These factors work together to regulate mitochondrial β-oxidation, which is critical for the extended lifespan of glp-1 mutants. Additionally, the conversion of saturated fatty acids to monounsaturated fatty acids is enhanced in germline-deficient nematodes, further promoting longevity.
2. glp-1 mutation and ARD can be stacked to extend lifespan
Mechanisms of life extension through genetic manipulation, such as glp-1 mutations, differ from those observed under natural conditions, like food deprivation and ARD. During food deprivation (FD), C. elegans naturally experience lifespan extension. However, glp-1 mutants do not exhibit further lifespan increases under these conditions, suggesting that somatic gonadal signaling and dietary restriction may share downstream mechanisms. Interestingly, glp-1 mutants subjected to ARD exhibit an extended lifespan (Fig. 1), suggesting that gonadal signaling and ARD utilize distinct, non-overlapping pathways to regulate longevity. Despite using distinct mechanisms, molecular studies have revealed some shared components between the germline pathway and ARD.Similar to glp-1 mutants that lack a proliferative germline, ARD animals require HLH-30 and DAF-16 for lifespan extension. HLH-30 plays a direct role in upregulating genes associated with lipid metabolism, such as fat-5, fat-6, nhr-80, and lip-3.However, reduced activity of DAF-12, SKN-1, and NHR-49, which are critical for lifespan extension in glp-1 mutants, has little effect on ARD-induced longevity, further highlighting the distinctiveness of these pathways.
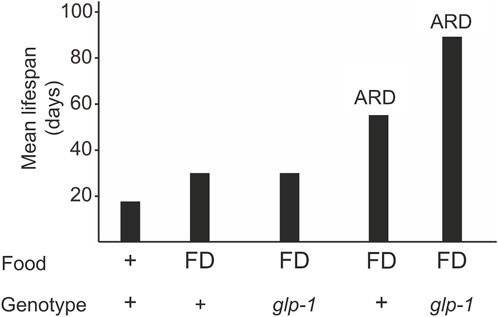
Figure 1. A glp-1 mutation and ARD additively extend longevity.
3. Germline ablation results in significant lifespan extension in hermaphrodites of most and rodioecious species
In hermaphrodites of and rodioecious species, senescence is associated with the production of excess yolk in the gut, leading to gut atrophy—a phenomenon not observed in virgin females of androdioecious species.In most hermaphroditic and male androdioecious species studied, germline or gonad removal leads to a substantial increase in lifespan. However, removing the entire gonad does not delay the onset of senescence, whereas germline removal does, suggesting an offsetting signal between gonadal tissues. Figure 2 illustrates the phylogeny of nematodes, highlighting androdioecious species in red. The arrow points to species that show significant lifespan extension after germline removal, either through Z2/Z3 ablation or glp-1 RNAi. In Pristionchus pacificus, a non-Caenorhabditis nematode, germline removal leads to the upregulation of genes involved in lipid metabolism, enrichment of DAF-16 targets, and downregulation of the insulin pathway—similar to observations in C. elegans. However, it remains unclear whether these molecular changes directly contribute to increased longevity. In contrast, germ cell ablation in the Oscheius species did not result in lifespan extension (Fig. 2), suggesting species-specific differences in the response to germline removal, possibly because it have not evolved mechanisms related to reproductive aging or death, which warrants further exploration.
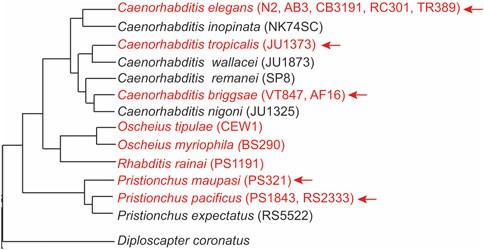
Figure 2. Germline ablation results in significant lifespan extension in hermaphrodites of most androdioecious species.
Conclusion
Nowadays, there is a growing interest in exploring pro-aging signals mediated by germ cells. Indeed, lifespan extension due to germ cell removal is not restricted to nematodes, but has been found in Drosophila, as well as in vertebrates such as fish.In germline-ablated C. elegans, transcriptional shifts and changes in lipid metabolism are key contributors to lifespan extension. The existence of analogous biochemical alterations in other species following germline removal, and the question of whether they operate through identical pathways, merits additional research.
References:
Andre Pires da Silva*, Rhianne Kelleher and Luke Reynoldson. Decoding lifespan secrets: the role of the gonad in Caenorhabditis elegans aging. Frontiers in Aging, 2024 Mar 26:5:1380016. doi:10.3389/fragi.2024.1380016.




