Unlocking Nature’s Digestive Signals: The Role of Bacterial Peptidoglycan in C. elegans Adaptation
2024-12-17 17:05
Keywords
Bacterial Peptidoglycan, Digestive Signaling, Food Adaptation, Host-Microbe Interaction
Contribution of Sunybiotech
This groundbreaking research was conducted in collaboration with Sunybiotech, a leader in genetic and biological research. Sunybiotech’s innovative tools and methodologies enabled precise manipulation of C. elegans genetics, contributing significantly to the discovery of how bacterial signals modulate host digestion.
The specific alleles generated by SunyBiotech:
PHX4067: syb4067[bcf-1p::bcf-1::3xflag::gfp(knock-in)]
Introduction
Survival in nature often hinges on adaptability, particularly in food consumption. For nematodes like C. elegans, which feed on diverse bacteria, the ability to digest various food sources is crucial. Yet, little is known about the signals that activate their digestive system. This study identifies bacterial peptidoglycan (PGN) as a critical signal enabling C. elegans to digest inedible food. It also reveals how PGN interacts with host proteins to suppress detrimental cellular stress and enhance food adaptation.
The Study: Objectives and Key Findings
Objectives
This research aimed to:
Explore whether bacterial peptidoglycan triggers the digestive system of C. elegans.
Decipher the molecular interactions enabling C. elegans to utilize low-quality or inedible food.
Uncover the role of PGN in promoting survival through dietary adaptability.
Key Findings
1. PGN as the "Good-Food Signal"
The researchers first asked whether PGN could activate the digestive system to process inedible food, such as Staphylococcus saprophyticus (SS). By supplementing SS with PGN extracted from E. coli, they observed a marked improvement in digestion and growth. Worms fed PGN-supplemented SS displayed a significant increase in body size and reduced intestinal bloating compared to controls fed SS alone.
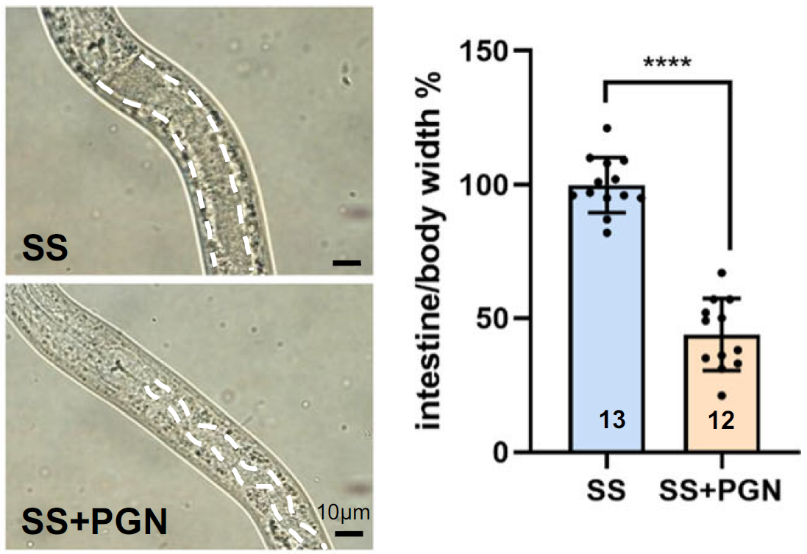
Figure 1: Reduced intestinal size and increased body size indicate a low ratio.
2. PGN Interacts with the Host Protein BCF-1
Next, the team examined whether PGN interacts with a specific protein in the intestine to activate digestion. Screening revealed that the glycoprotein BCF-1 binds directly to PGN and is critical for its function (Fig.2a). Worms lacking BCF-1 or exposed to PGN-deficient bacteria (ΔycbB) showed impaired growth, while wild-type worms supplemented with PGN exhibited robust digestion and development (Fig.2b). This finding raised another question: How does BCF-1 facilitate digestion at the molecular level?
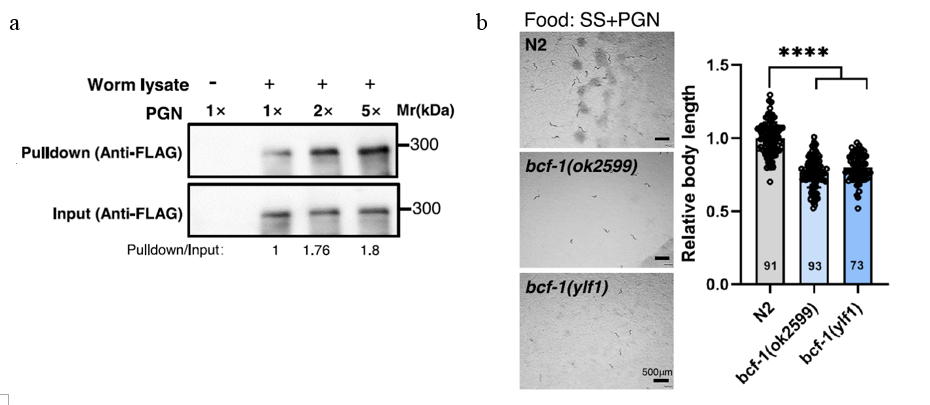
Figure 2: Worms lacking BCF-1 or exposed to its absence show impaired growth.
3 Suppression of Mitochondrial Stress Enhances Digestion
Then, the researchers discovered that BCF-1 suppresses the mitochondrial unfolded protein response (UPRmt), a stress pathway that inhibits digestion. PGN supplementation reduced UPRmt activation, improving digestion and growth. However, this effect was absent in BCF-1 mutants, suggesting that PGN acts through BCF-1 to suppress stress. With BCF-1 and UPRmt identified as key players, the team delved deeper into the adaptive significance of this interaction.
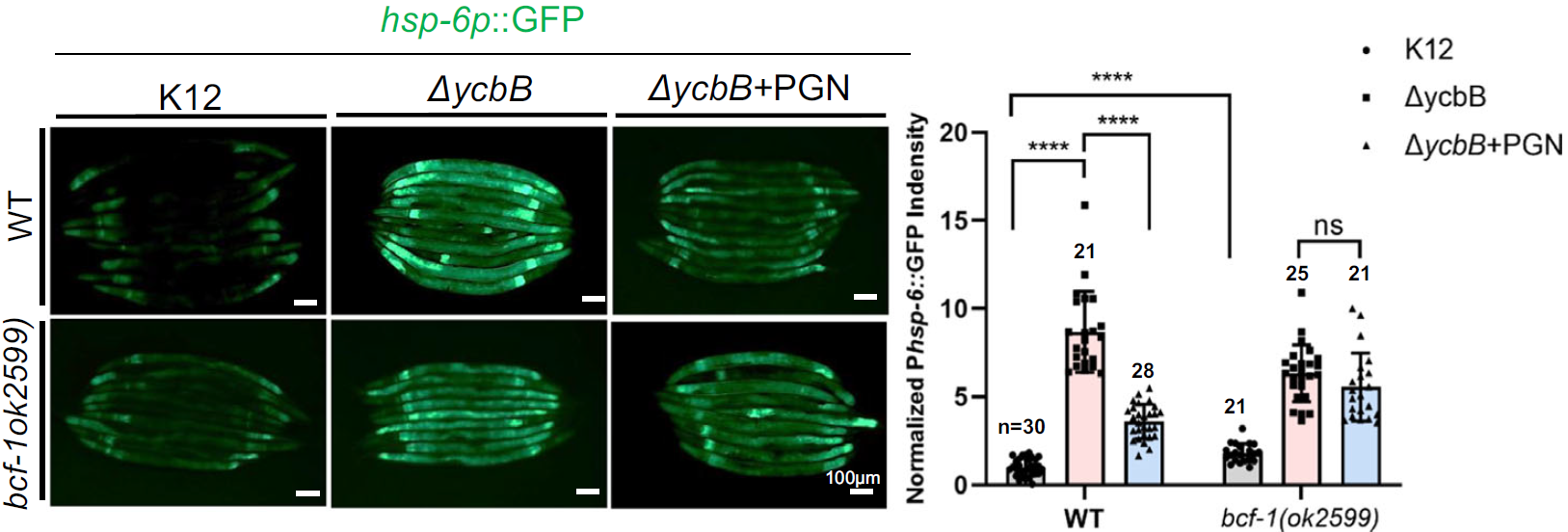
Figure 3: BCF-1 suppresses the mitochondrial unfolded protein response.
4. PGN Drives Adaptation in Complex Environments
In natural settings, food sources are diverse and often low-quality. The researchers simulated these conditions by feeding worms a mix of bacteria, including SS and low-quality options. PGN-enabled digestion proved critical for population expansion under such conditions. Worms fed mixed bacterial diets supplemented with PGN produced more offspring than those without PGN, demonstrating its role in adaptation.
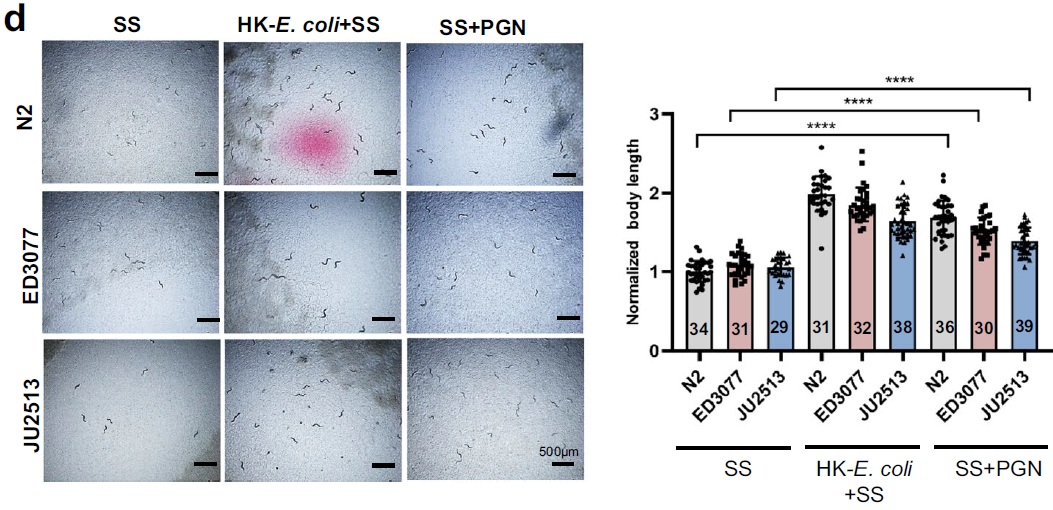
Figure 4: Worms fed mixed bacterial diets supplemented with PGN produce more offspring.
5. Neuropeptide NLP-3 Links the Gut and Brain
Finally, the study showed that the PGN-BCF-1 interaction regulates neuropeptide NLP-3 release, connecting intestinal signaling to neuronal control of digestion. Knocking down NLP-3 in BCF-1 mutants partially restored digestion, highlighting its role in the gut-brain axis.
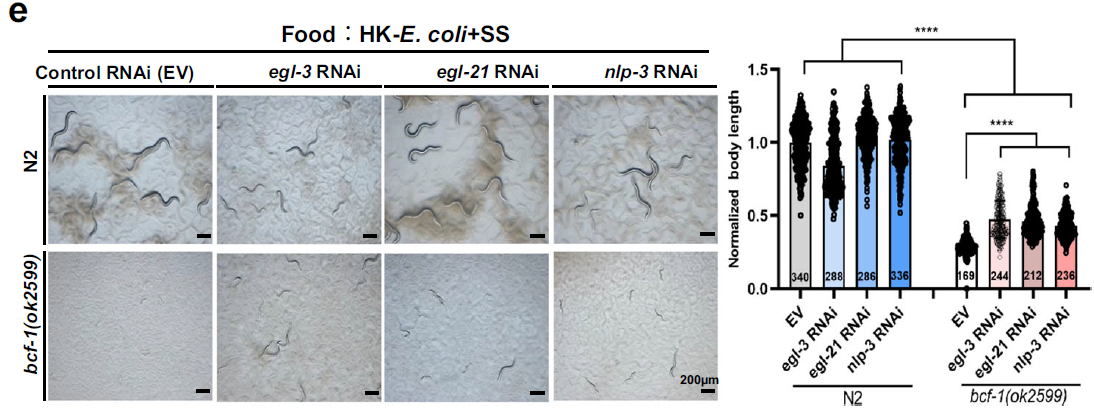
Figure 5: Knocking down NLP-3 in BCF-1 mutants partially restores digestive function.
Conclusion
This study illuminates how bacterial PGN acts as a "good-food signal," activating digestion and promoting ecological adaptation in C. elegans. By interacting with BCF-1, PGN suppresses mitochondrial stress and enables worms to thrive on diverse food sources. These findings not only deepen our understanding of host-microbe interactions but also open avenues for exploring microbial influences on digestion in other organisms.
References
Hao, F., Liu, H., & Qi, B. (2024). Bacterial peptidoglycan acts as a digestive signal mediating host adaptation to diverse food resources in C. elegans. Nature Communications, 15, 3286. DOI:10.1038/s41467-024-47530-y.







