The Sleep-Active Neuron: A Guardian of Survival Beyond Slumber
2025-01-14 15:08
Keywords: RIS, FLP-11, sleep behavior, stress survival, sleep-active neuron
Introduction
Sleep has been a fascinating topic in exploring the mysteries of human and animal behaviour. It is orchestrated by specialized neurons that drive behavioral quiescence and promote physiological restoration. Despite decades of study, the exact mechanisms linking sleep to survival remain elusive. An article published in PLOS GENETICS shed light on how sleep-active neurons can promote survival, even when sleep behaviour is disturbed. Using Caenorhabditis elegans as a model, this study focused on retained in sleep (RIS) neurons, which not only drive sleep behavior but are also essential for surviving stressors like starvation and trauma. The groundbreaking findings in this study not only improve our understanding of sleep function, but also provide new directions for future sleep research and treatment. Let's unravel the mystery of this scientific discovery and explore the complex interactions between sleep and survival.
Contribution of SunyBiotech:
SunyBiotech constructed a number of specific C. elegans strains for this study, which are essential for studying the function of RIS neurons:
PHX1433: flp-11(syb1433 [flp-11-SL2-egl-23cDNA(A383V)-linker-mKate2]) X
PHX1445: aptf-1(gk794) II; flp-11(syb1445[flp-11-SL2-unc-58(L428F)-linker-mKate2]) X
PHX1464: flp-11(syb1464[flp-11-SL2-egl-23cDNA(L229N)-linker-mKate2]) X
PHX2193:flp-11(syb2193[flp-11b-SL2(gpd-2)-mKate2-linker-twk-18(e1913)]) X
PHX2493:lgc-38(syb2346[flp-11p::dpy-10 site::flp-11 3’UTR], syb2493[ReaChR-linkermKate2]) III.
PHX3190: lgc-38(syb2346[flp-11p::dpy-10 site::flp-11 3’UTR], syb3190[unc-58(e665)-linker
(GSGSGSGSG)-mKate2]) III
PHX4110: lgc-38(syb2346[flp-11p::dpy-10 site::flp-11 3’UTR], syb4110[unc-58gf-CAI-
1.0-linker(GSGSGSGSG)-mKate2]) III
PHX4416: aptf-1(gk794) II; flp-11(syb1445 syb4416) X
1. Dual roles of RIS neurons
To manipulate the activity of RIS neurons, the researchers expressed mutant ion channels, including the potassium channels twk-18gf and egl-23gf, and the sodium channel unc-58gf. This approach allowed precise control over RIS neuron activity. The study revealed that low levels of unc-58gf increased calcium transients in RIS, enhancing sleep behavior. Conversely, high levels inhibited these transients, locking RIS activity at a constant, elevated state during L1 arrest. This resulted in RIS activity being locked at a high but constant level, causing almost complete loss of sleep behaviour (Fig. 1A and C) but increased survival (Fig. 1B). This implies that RIS neurons can provide survival benefits even when behavioral sleep is disturbed. The ability of RIS neurons to promote survival independently of sleep behaviour suggests that promotion of sleep behaviour and survival are separable functions of the RIS, which are normally coupled during sleep but can be uncoupled when the RIS is strongly activated or sleep behaviour is impaired.
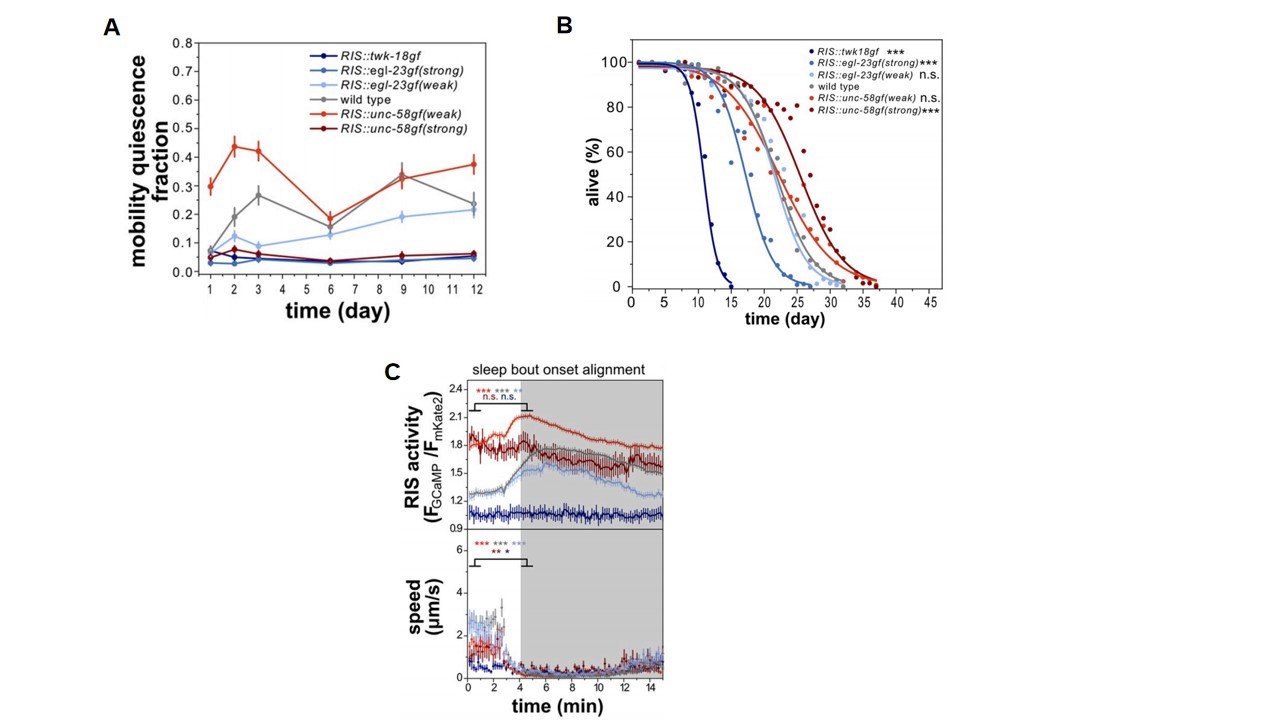
Figure 1. Dual roles of RIS neurons
2. Impact of sleep disruption on survival
Beyond ion channel alterations, sleep can also be disrupted by optogenetic activation or blue light stimulation as a way to mimic changes in RIS neuron activity under sleep-disrupting conditions. Research demonstrates that prolonged optogenetic activation significantly elevates RIS activity, which tends to enhance survival (Fig. 2A and B). Similarly, lethal blue light stimulation overactivated RIS, resulting in increased survival (Fig. 2C and D). These findings suggest that RIS acts as a protective neuron, responding to stressful stimuli by promoting survival. Importantly, this survival-promoting function may operate independently of behavioral sleep.
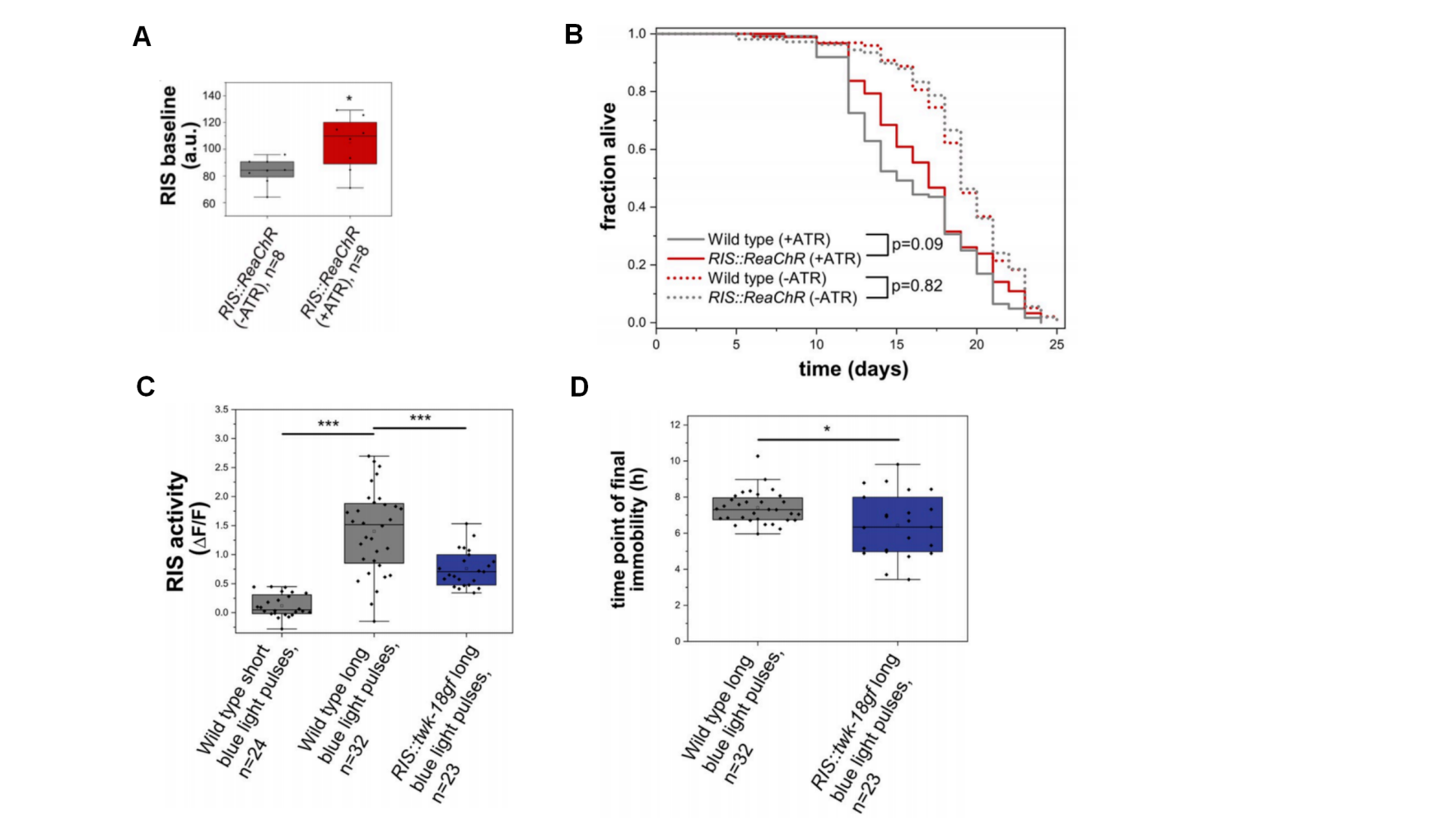
Figure 2. Impact of sleep disruption on survival
3. Role of FLP-11 neuropeptides
While RIS governs both sleep and survival, what is the key transmitter enabling these functions? It was found that FLP-11 is a group of neuropeptides of RIS that presents the main transmitters required for RIS. In FLP-11 deletion mutants including flp-11(-) and RIS::unc-58gf(strong), flp-11(-), showed reductions in both sleep and survival (Fig. 3A and B). These findings suggest that FLP-11 is crucial for inducing sleep behavior and supporting survival during starvation, with potentially distinct roles downstream of RIS. This may be one of the mechanisms by which RIS neurons function between sleep behaviour and survival.
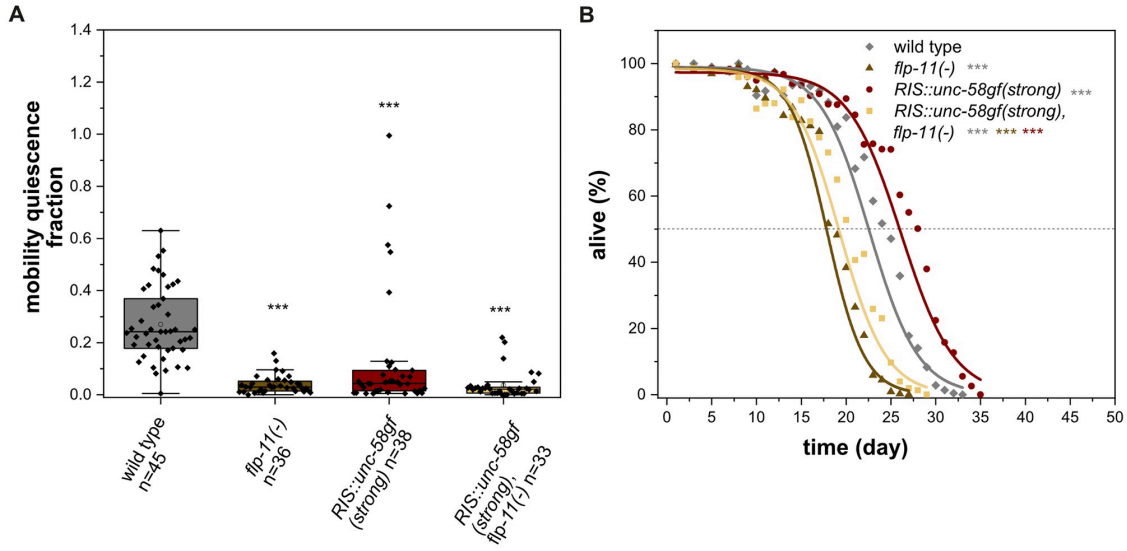
Figure 3. Role of FLP-11 neuropeptides
Conclusion
The dual role of RIS neurons highlights their complexity, functioning as both sleep inducers and survival promoters. Typically, these two functions are intertwined, but they can be uncoupled under circumstances of intense RIS activation or stress, such as trauma. This discovery underscores that RIS neurons can safeguard survival even in the absence of sleep behavior. As a key neurotransmitter of RIS, FLP-11 neuropeptides may regulate sleep behavior and survival responses through different receptors and downstream pathways after RIS activation. A detailed study of this mechanism could provide a new perspective for understanding the physiological roles of sleep. Sleep science is a rapidly evolving field. Understanding the functions of sleep-active neurons could help develop treatments to assist patients with sleep disorders or individuals under high-stress conditions to maintain health and survival.
References
Busack I, Bringmann H. A sleep-active neuron can promote survival while sleep behavior is disturbed. PLOS Geneticst. 2023 Mar 14;19(3):e1010665.





